Describe the Construction Working and Useful of Dry Cell
This versatility makes it suitable for portable equipment. The carbon rod is surrounded by a mixture of carbon particles and a chemical called ammonium chloride.
Dry Cell Definition Working Principle And Types Of Dry Cell
It is white in appearance and works as a negative terminal of the cell.

. Around this plate there is an outer jacket of glass which has a side inlet through which pure and dry hydrogen gas is bubbled at one atmosphere pressure. Describe the construction working and usefulness of a dry cell with the help of a diagram. Dry cell batteries are different from wet cells because their electrolytes are contained in a low-moisture paste while a wet cell has electrolytes contained in a liquid hence the difference in names.
The battery which uses sponge lead and lead peroxide for the conversion of the chemical energy into electrical power such type of battery is called a lead acid battery. Construction of dry cell. A dry cell is an electrical power generator dependent on chemical reactions.
Explain the effect of overcharging lead acid battery Make accurate qualitative statements about battery systems. A carbon rod is placed at the centre with a brass cap which acts as the positive electrode. Describe the construction and working of a hydrogen fuel cell A fuel cell is a lot like a battery.
Sal Amminiac is a mineral composed of Ammonium Chloride NH 4 Cl. The electrolyte uses is a moist paste of ammonium chloride. Fine-grained manganese dioxide MnO 2 powder mixed with coal dust is molted to.
The cell pushes the electrons to fluid from one end to the other when the two electrodes are bound in a closed way. This ScienceStruck post provides the history definition composition uses and recycling process of. I The standard hydrogen electrode SHE consists of a glass tube at the end of which a piece of platinised platinum foil is attached as shown in Fig.
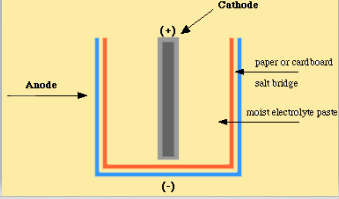
A dry cell consists of a metal container in which a low moisture electrolyte paste covers the graphite rod or a metal electrode. It is used as secondary reference electrode to determine the standard potentials of the electrode. A commonly used dry cell is the zinc carbon cell or the dry Leclanche cell.
A rubber bung carrying a thin glass tube with a. This is surrounded by a mixture of manganese dioxide and charcoal in a muslin bag. Unlike a wet cell a dry cell can operate in any orientation without spilling as it contains no free liquid.
The life of the dry cell is longer than cells using liquid. Distinguish between primary secondary battery types. A dry cell consists of a zinc container whose base acts as the negative electrode.
In the question we asked to describe the construction working and also the usefulness of a dry cell with the help of a diagram. A battery depolarizer takes up electrons during discharge of the cell and hence depolarizer is always an oxidizing agent. The electrolyte in this cell battery contains very little moisture to allow the passage of current through it.
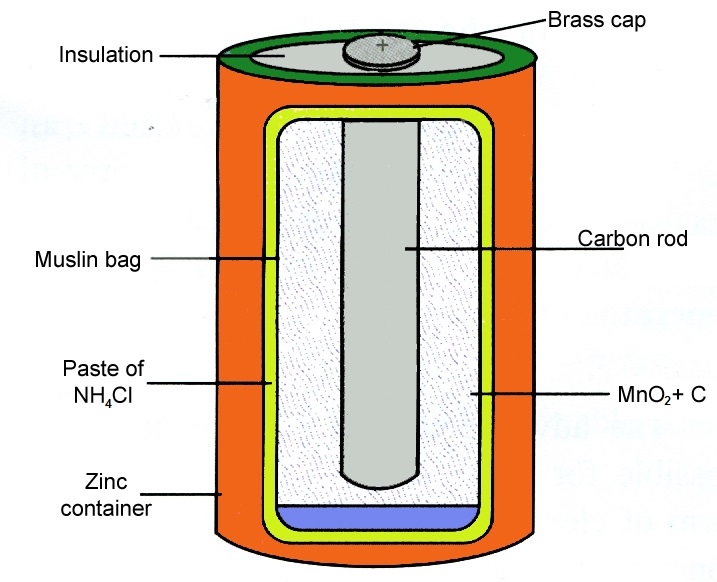
The carbon rod placed at the centre with a brass cap acts as the positive electrode. This drum contains all materials of the battery and it also serves as the cathode of the battery. These electrodes do not obstruct light to reach the thin p-type layer.
1 Construction. The container also serves as the negative terminal in the centre. The positive terminal of the battery is projected from the top of this drum.
It has two electrodes where the reactions take place and an electrolyte which carries the charged particles from one electrode to the other. By comparison the first wet-cell batteries were typically fragile glass containers with lead rods hanging from. The electrode consists of a glass tube provided with a bent side tube and another side tube B.
The body of the battery is made of a hollow steel drum. Learning objectives Demonstrate an understanding of the meaning of the terms cell battery charging recharging separator fuel cell. Understanding the Working Principle and Uses of a Dry Cell Battery.
The outer metal cover is made up of Zinc metal. A carbon graphite rod with a metal cap serves as a positive terminal. This is surrounded by a mixture of manganese dioxide and charcoal in a muslin bag.
Generally the metal container will be zinc whose base acts as a negative electrode anode and a carbon road acts as a positive electrode cathode. A dry cell consists of the following components 1. Basically a dry cell could be categorized as a type of electric battery that can be commonly used for portable electrical devices.
A dry cell consists of a container made up of zinc metal. A dry cell consists of a zinc container whose base acts as the negative electrode. Usefulness of dry cell.
They are handy and portable. A little H g is placed at the bottom of the dry glass tube. Steps 4 to 7 are repeated by using two dry cells connected in series.
The terminals of the dry cell are reversed and connected back to the Y-input terminals. The voltages of the dry cell calculated from the two settings are compared. The dry cell battery is one of the most commonly used types including AA 9-volt and watch batteries.
Depolarizer is basically a substance used in a cell to prevent build-up of hydrogen gas bubbles. A dry cell has the electrolyte immobilized as a paste with only enough moisture in it to allow current to flow. Describe construction working of some battery systems fuel cells.
It acts as the positive terminal of the cell. Calomel electrode is a metal-sparingly soluble salt electrode. Because of this electric charge is produced on the two terminals of the cell and electric current flows in the circuit.
The position of the bright spot or line on the screen is observed and compared. In Dry Cell electrolyte is paste of sal ammoniac and Zinc Chloride. The electrolyte uses is a moist paste of ammonium chloride.
The Y-gain is set back to 1 V div. A dry cell battery is a type of chemical battery that uses an electrolyte which is in the immobilized state. A dry cell consists of a zinc container and its base works as the negative electrode.
Description of a dry cell. The carbon rod placed at the centre with a brass cap acts as the positive electrode. The electron flow allows the electrons to flow into the closed system.
This is surrounded by a mixture of manganese dioxide and charcoal in a muslin bag. The electrolyte uses is a moist paste of ammonium chloride. Chemical reactions take place between the electrolyte zinc container and graphite rod.
The lead acid battery is most commonly used in the power stations and substations because it has higher cell voltage and lower cost. Working of dry cell. A solar cell is basically a junction diode although its construction it is little bit different from conventional p-n junction diodesA very thin layer of p-type semiconductor is grown on a relatively thicker n-type semiconductorWe then apply a few finer electrodes on the top of the p-type semiconductor layer.
Construction of Alkaline Battery.

Dry Cell Structure Working Chemical Reactions Its Applications

Dry Cell Structure Working Chemical Reactions Its Applications
Explain The Construction Of A Dry Cell With An Appropriate Diagram Physics Topperlearning Com 36gl66zz
No comments for "Describe the Construction Working and Useful of Dry Cell"
Post a Comment